Investigating Abnormal Chromosome Tolerance in Wild Yeast Cells
Hose et al (2020) researched the genetic basis of aneuploidy tolerance in wild yeast in a recent edition of Elife.
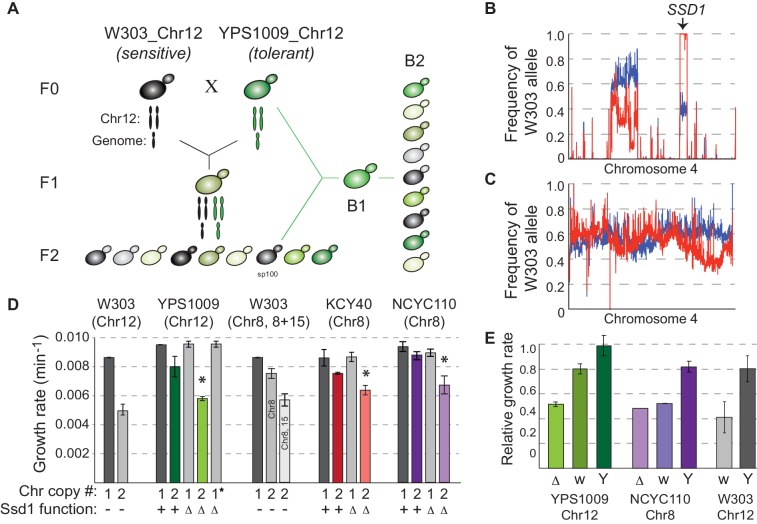
Aneuploidy, or the abnormal chromosome numbers in a cell, is a harmful condition in developmental stages of wild yeast, yet is also common in plant cancers and pathogenic fungi. It is interesting to note that aneuploidy tolerance varies; for instance, researchers found that some wild isolates of baker’s yeast can tolerate chromosome amplification, while laboratory strains cannot.
To study the genetic basis of how well wild yeast can tolerate aneuploidy, researchers mapped the genetic basis to Ssd1, an RNA-binding translational regulator that functions in wild strains but is defective in a laboratory strain “W303.”
Researchers found that aneuploidy tolerance is enabled via a role for Ssd1 in mitochondrial physiology, such as binding and regulating nuclear-encoded mitochondrial mRNAs.