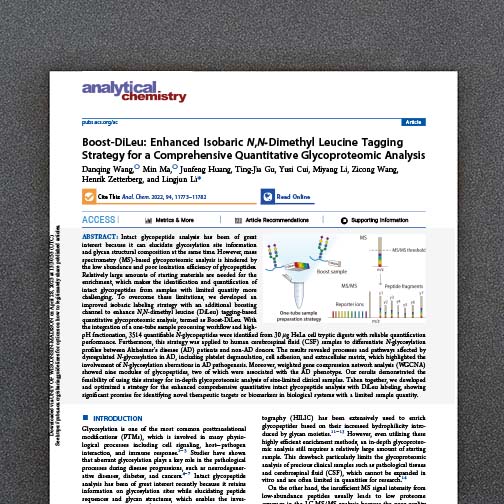
Isobaric chemical labeling approaches have enabled a new generation of multi-plexed quantitative proteomic studies. The strategy labels peptides primary amines with isotope-coded mass tags that are indistinguishable from each other during normal mass measurement; however, upon dissociation and tandem mass spectrometry unique reporter ions are produced and used for quantification. We are developing a new dimethylated leucine (DiLeu) scaffold for isobaric tag technology. The DiLeu platforms incorporates the same mass defect concepts as our NeuCode labels and has potential to profoundly increase the number of samples that can be co-analyzed in a single LC-MS/MS experiment.