Transgenic animal models demonstrate that mice have similar COPD-related skeletal muscle dysfunction as humans
Patients with chronic obstructive pulmonary disease (COPD), a disease that is characterized by obstructed airflow to the lungs and is associated with long-term exposure to cigarette smoke, often also develop skeletal muscle dysfunction. Overall, the comorbidity of COPD and skeletal muscle dysfunction is associated with outcomes such as poor health and mortality.
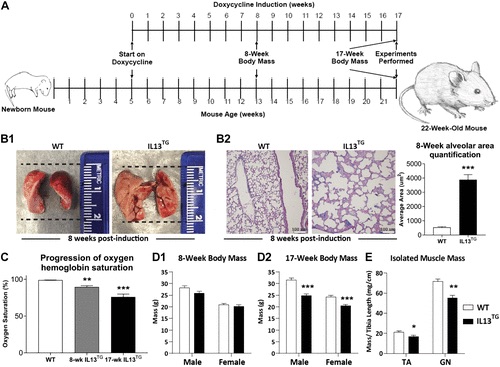
While some research has suggested that skeletal muscle dysfunction may be the result of COPD-related conditions, such as protein degradation and metabolic disruption, there is still poor understanding on what mechanisms would regulate these processes, as there is little to no research on a validated animal model of pulmonary emphysema. Therefore, Balnis et al (2020) sought to use such a model based on inducible UL-13-driven pulmonary emphysema (IL-13TG) to study the mechanisms of skeletal muscle dysfunction.
Using a transgenic mouse model, researchers found that the skeletal muscles of emphysematous mice are similar to those developed by human patients with COPD. For instance, both groups develop muscular atrophy and have decreased oxygen consumption. Within skeletal muscles, both groups also had downregulated ATP binding and bioenergetics.
Researchers concluded that transgenic animal models of COPD are useful to understand skeletal-muscle dysfunction in humans.